Clinical Practice Research Datalink
Transforming a paper-based Government clinical research agency into a digital-first organisation.
About CPRD
The Clinical Practice Research Datalink (CPRD) supports clinical research using anonymised electronic health records from over 30 million UK patients, collected over nearly 30 years.
As part of the Medicines and Healthcare products Regulatory Agency (MHRA), CPRD has been providing anonymised primary care records for public health research since 1987. This data, used by academics, regulators, and the pharmaceutical industry, has led to over 1,500 publications, improving drug safety, best practices, and clinical guidelines.
Starting with the rejuvenation of their ISAC application process, our ongoing work has extended into the development of a customer portal, a new Learning Management System, and an AI-driven offering to create synthetic data sets for their users. We are now also creating a chatbot for the website – answering FAQs and directing people to the right place on the site.
The challenge
CPRD faced the overarching challenge of transforming a complex, paper-based system into a streamlined, digital-first process. The goal was to digitise all aspects of their service, reducing the need for human intervention and making it easier for researchers and internal staff to use the system efficiently.
The wider digital transformation work aimed to increase the number of users, simplify the application process, and create a self-service, automated system. This will allow CPRD to manage its services with minimal manual involvement, ultimately saving time and resources.
Our solution
To address CPRD’s challenges, we implemented a comprehensive digital transformation strategy, focusing on five key areas:
Streamlining Online Application Processes
We began by reworking the Independent Scientific Advisory Committee (ISAC) application process. The objective was to make it more user-friendly, enabling researchers to track their application status and receive timely feedback. On the back-end, we streamlined the process for reviewers, integrating the entire application workflow into a single system to ensure all stages were completed within specific timelines. This process followed GDS best practices and involved a discovery phase, alpha prototyping, and rigorous beta testing.
Creation of a Customer Portal
After having successfully streamlined the application process, we are now developing a new customer portal for CPRD. It will replace their basic data transfer system with a secure, user-friendly platform. This portal will allow users to manage their organisation’s details, assign roles, download previous applications, and securely access requested data. It’s designed to be a one-stop shop where users can manage all aspects of their interactions with CPRD, from application submission to data retrieval. The portal will not only enhance security but also significantly improve efficiency by allowing users to re-access and amend previous data requests easily.

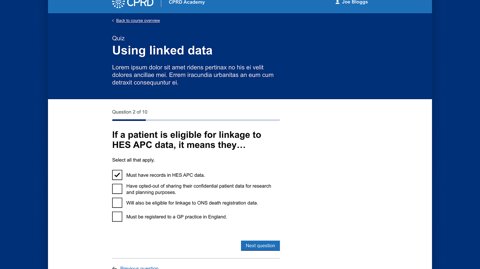
Learning Management System (LMS)
To support users and internal staff, we are also building a Learning Management System (LMS) integrated into the customer portal. This LMS will provide compulsory training courses to help users create successful applications and navigate the system. For CPRD staff, it will offer training on system usage, application review, and feedback processes, ensuring that both external users and internal teams are well-equipped to use the digital platform effectively.
Additionally, we are redesigning CPRD’s educational tutorial videos, modernising their content to make it more engaging and effective. We are utilising AI to create voiceovers for these videos, ensuring that they are accessible and easy to follow for all users. This video service will further enhance the training experience, making it more dynamic and user-friendly.
Creation of a Chatbot for the Website
To further improve user experience and reduce the need for direct human support, we are developing a chatbot for CPRD’s website. This chatbot is designed to answer the most frequently asked questions and guide users to the relevant sections of the site. By addressing common queries and helping users find the information they need quickly, the chatbot will free up CPRD staff to focus on more complex queries and tasks. It’s another essential step towards making CPRD’s services more accessible and user-friendly.
AI-Driven Offering to Create Synthetic Data Sets
Finally, in collaboration with CPRD, we are developing a cutting-edge system that leverages AI to create synthetic data sets from smaller, real data sets. This offering is particularly valuable for researchers who need larger data sets for their studies but only have access to limited real-world data. By using AI to expand these data sets, researchers can conduct more comprehensive studies without compromising on data quality. Our role includes creating the front-end system that allows users to request, process, and securely receive these synthetic data sets. This innovative solution will significantly enhance CPRD’s research capabilities.
Reading Room just ‘got it’ instantly. My experience has been one of collaborative working with some really talented individuals who take pride in producing a first class product.
Mulling over a digital challenge?
We're here to help you transform ideas into impactful solutions. Let’s explore new approaches and uncover the right strategies to bring your vision to life.